22+ enthalpy reaction calculator
Example of First-Order Reaction. The term standard state is used to describe a reference state for substances and is a help in thermodynamical calculations as enthalpy entropy and Gibbs free energy calculations.

Example How To Calculate Enthalpy Change Of A Reaction Youtube
Electron Gain Enthalpy - Negative electron gain enthalpy the difference between electron gain enthalpy and electron affinity.

. Metalorganic frameworks MOFs are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one- two- or three-dimensional structures. Two general conceptual comments can be made about the results of the two sample calculations above. The heat cap acity C of a body of matter is the quantity of heat q it absorbs or releases when it experiences a temperature change ΔT of 1 degree Celsius or equivalently 1 kelvin.
This Gibbs free energy calculator is just perfect if youre looking for a tool that estimates whether or not a reaction can happen spontaneouslyThe Gibbs free energy equation AKA. 2R-X 2Na R-R 2Na X. The holding will call into question many other regulations that protect consumers with respect to credit cards bank accounts mortgage loans debt collection credit reports and identity theft tweeted Chris Peterson a former enforcement attorney at the CFPB who is now a law.
Heat capacity is determined by both the type and amount of substance that absorbs or. 27315 K 101325 kPa is 22413 962 x 10-3 m3 mol-1 with a standard uncertainty of 0000013 x 10-3 m3 mol-1 2. For a gas the standard state is as a pure gaseous.
Thank you so much. During a chemical reaction absorption and evolution of energy take place. The enthalpies of hydration for potassium and chloride are -322 and -363 kJmol respectively.
ASCII characters only characters found on a standard US keyboard. What is Sucrose C 12 H 22 O 11. The variables include acceleration a time t displacement d final velocity vf and initial velocity vi.
This non-reducing disaccharide has a chemical formula of C 12 H 22 O 11. When zinc reacts with strong sodium hydroxide it gives sodium zincate and hydrogen gas. Metal oxides are produced when metals burn in the presence of oxygen.
Water is the chemical substance with chemical formula H 2 O. The enthalpy of crystallization for KCl is -715 kJmol. A few examples of chemical change are digestion of food burning of coal rusting etc.
Now one must find if the entropy is greater than zero to answer the question. The calculator below uses the formula to convert liters to moles and. Adding i and ii the atomic mass of the given sample is.
That means the impact could spread far beyond the agencys payday lending rule. The general form of the Wurtz reaction equation can be written as follows. Consider the hydrolysis of ethyl acetate during the hydrolysis the concentration of ethyl acetate is 002 molL whereas the amount of water is 20 molL as the process of hydrolysis involves a large amount of waterLet us say the process of hydrolysis attains completion in.
The second isotope has an atomic mass of 3696590 and has an abundance of 2422. Some examples of physical change are freezing of water melting of wax boiling of water etc. The order of reaction does not depend on the stoichiometric coefficients corresponding to each species in the balanced reaction.
ΔH ΔG S Definitions of standard states. Sucrose is commonly referred to as table sugar or cane sugar. In the reaction described by the equation CH 4 2O 2 CO 2 2H 2 O the stoichiometric coefficient of O 2 and H 2 O is 2 whereas that of CH 4 and CO 2 is 1.
The Physics Classroom serves students teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Kinematic equations relate the variables of motion to one another. This can easily be observed in a.
Ayushi September 14 2019 at 512 pm. 6 to 30 characters long. Here is an example to help you understand the concept more clearly.
The Shopping Cart is. From these values estimate the enthalpy of solution for KCl. The delta G equation combines the enthalpy vs.
Each equation contains four variables. While the FREE version does all the above teachers with a Task Tracker subscription can take things a step further. The organic ligands included are sometimes referred to as struts or linkers one example being 14-benzenedicarboxylic acid BDC.
In a C 12 H 22 O 11 molecule the fructose and glucose molecules are connected via a glycosidic bond. We now introduce two concepts useful in describing heat flow and temperature change. Atomic mass of each isotope x Abundance 100 349688507578 2650 i 369659002422 895 ii Step 2.
These metal oxides are basic in nature. If the enthalpy is negative then the reaction is exothermic. The first isotope has an atomic mass of 3496885 and has an abundance of 7578.
Not all the metals react with bases and when they do react they produce metal salts and hydrogen gas. The enthalpy of hydration for KCl is estimated to be Delta H_hyd -322 -363 -685 kJmol nonumber. They can modify our pre-made problem sets write their own problems with our easy-to-use Problem Builder and use the Calculator Pad to design their own program that expresses their emphasis on the use of mathematics in Physics.
The reaction order of a chemical reaction is always defined with the help of reactant concentrations and not with product concentrations. If values of three variables are known then the others can be calculated using the equations. The superscript degree symbol indicates that substances are in their standard states.
Trends in ionization enthalpy in a group. The solution of the problem involves substituting known values of G 6673 x 10-11 N m 2 kg 2 m 1 598 x 10 24 kg m 2 70 kg and d 639 x 10 6 m into the universal gravitation equation and solving for F gravThe solution is as follows. Water is the medium through which all essential vitamins and minerals are transported in the bodies of living organisms owing to its ability to dissolve a wide range of substances.
Sucrose is a molecule composed of two monosaccharides namely glucose and fructose. Using the entropy of formation data and the enthalpy of formation data one can determine that the entropy of the reaction is -421 JK and the enthalpy is -412 kJ. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
More formally a metalorganic framework is a coordination. A reaction related to the Wurtz Reaction in which aryl halides are used instead of alkyl halides is often called the Wurtz-Fittig reaction and is a very important named reaction in organic chemistry. Importance of Water in Living Organisms.
This page demonstrates the process with 20 sample problems and. The upcoming paragraphs tell you how to calculate Gibbs free energy provide the Gibbs free energy units and give the. The enthalpy change for the combustion of 1 mole of methane gas is given in the table as a negative value.
The total number of atoms of an element present in a species in a balanced chemical equation is equal to the product of the stoichiometric coefficient and the number of atoms of the. Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers. Generally physical changes do not involve the production of energy.
Must contain at least 4 different symbols. The value of the order of reaction can be in the form of an integer or a fraction. The first ionization enthalpy of elements decreases as we move down in a group.
While moving down in a group the atomic number increases and the number of shells also increases. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Outermost electrons are far away from the nucleus and thus can be removed easily.
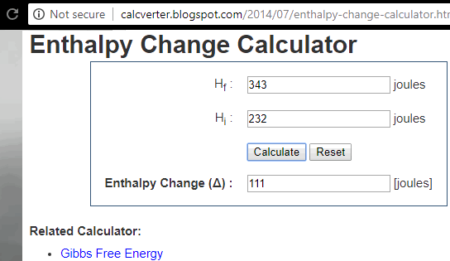
4 Free Online Reaction Enthalpy Calculator Websites

Stable Carbenes Chemical Reviews
Enthalpy Calculator 100 Free Calculators Io

Energetics Connect With Chemistry World

4 Free Online Reaction Enthalpy Calculator Websites

2016 J2 H2 Chem Prelim Updated Pw Pdf Hydroxide Solubility

Enthalpy Calculator

Enthalpy Change Chemblogsxc

Enthalpy Change Of Reaction Formation Thermochemistry Calorimetry Practice Problems Youtube

Low Temperature Fischer Tropsch Fuels From Syngas Kinetic Modeling And Process Simulation Of Different Plant Configurations Sciencedirect

Enthalpy

Enthalpy

Stable Carbenes Chemical Reviews

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Class 12 Chemistry Worksheet On Chapter 8 The D F Block Elements Set 4
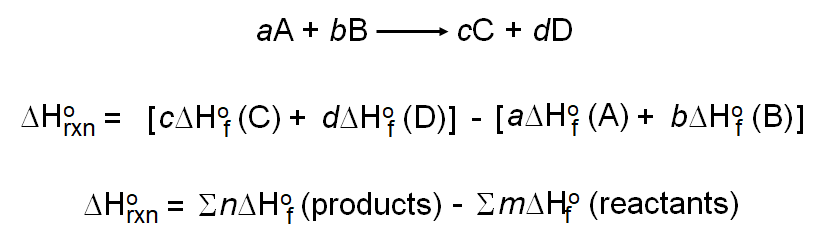
Chm1045 Enthalpy Lecture